

Victor Viau
Professor
PhD (McGill University)
MSc (McGill University)
BSc (Concordia University)
Email: victor.viau@ubc.ca
Office: 604-822-3899
Lab: 604-822-9736
Lab Website: viaulab.med.ubc.ca
The hypothalamic-pituitary-adrenal (HPA) axis is an important hormonal system in man and rodents, which ultimately controls secretion of glucocorticoids from the adrenal gland: cortisol in humans, and corticosterone in rats. Stress-induced activation of the HPA axis resulting in acute elevations in circulating glucocorticoids levels protect the organism from physiological insult by regulating a variety of physiological processes. For example, they provide adequate substrate for increased metabolic need and help to sustain blood pressure and depress immune function. On the other hand, chronic elevations in glucocorticoids produced by repeated stress exposure have been implicated in the pathogenesis of several forms of systemic, neurodegenerative, and affective disorders.We have evidence showing that testosterone acts centrally to inhibit stress-induced HPA activity and corticosterone release in the rat. Using a functional neuroanatomical approach assisted by tract-tracing, in-situ histochemical, and early-gene techniques, our goal is to reveal the routes, neurotransmitters, and cellular mechanisms by which testosterone alters circuits in the brain conveying stress-related information. Because gonadal steroid release in both males and females varies as a function of reproductive and social status, this research will lay the groundwork for future studies aimed at understanding the central bases of social- and gender-based differences in stress reactivity.
- Freitas-Andrade M, Bechberger JF, MacVicar BA, Viau V, Naus CC (2017) Pannexin1 knockout and blockade reduces ischemic stroke injury in female, but not in male mice. Oncotarget. 8(23):36973-36983.
- Radley J, Morilak D, Viau V, Campeau S (2015) Chronic stress and brain plasticity: Mechanisms underlying adaptive and maladaptive changes and implications for stress-related CNS disorders. Neurosci Biobehav Rev. 58:79-91.
- Gray M, Innala L, Viau V * (2014) Central vasopressin V1A receptor blockade alters patterns of cellular activation and prevents glucocorticoid habituation to repeated restraint stress exposure. International Journal of Neuropsychopharmacology 10: 1-11.
- Goel N, Innala L, Viau V* (2014) Sex differences in serotonin (5-HT) 1A receptor regulation of HPA axis and dorsal raphe responses to acute restraint. Psychoneuroendocrinology 40: 232-241.
- Bingham B, Wang NX, Innala L, Viau V*. (2012) Postnatal aromatase blockade increases c-fos mRNA responses to acute restraint stress in adult male rats. Endocrinology 153:1603-1608.
- Shafiei N, Gray M, Viau V, Floresco SB (2012) Acute stress induces selective alterations in cost/benefit decision-making. Neuropsychopharmacology 37: 2194-2209.
- Hill MN, McLaughlin R, Pan B, Fitzgerald M, Roberts C, Lee T, Karatsoreos I, Mackie K, Viau V, Pickel V, McEwen BS, Liu QS, Gorzalka B, Hillard C. (2011) Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. Journal of Neuroscience 31: 10506-10515.
- Bingham B, Myung C, Innala L, Gray M, Anonuevo A, Viau V* (2011) Androgen receptors in the posterior bed nucleus of the stria terminalis increase neuropeptide expression and the stress-induced activation of the paraventricular nucleus of the hypothalamus. Neuropsychopharmacology 36:1433-1443.
- Bingham B, Gray M, Sun T, Viau V* (2011) Postnatal blockade of androgen receptors or aromatase impairs the expression of stress hypothalamic-pituitary-adrenal axis habituation in adult male rats. Psychoneuroendocrinology 36:249-257.
- Williamson M, Bingham B, Gray M, Innala L, Viau V*. (2010) The medial preoptic nucleus integrates the central influences of testosterone on the paraventricular nucleus of the hypothalamus and its extended circuitries. Journal of Neuroscience 30:11762-11770.
- Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TTY, Gray M, Hillard CJ, Gorzalka BB, Viau V*. (2010) Endogenous cannabinoid signaling is essential for stress adaptation. Proceedings of the National Academy of Sciences 107:9406-9411.
- Gray M, Bingham B, Viau V* (2010) A comparison of two repeated restraint stress paradigms on hypothalamic-pituitary-adrenal axis habituation, gonadal status and central neuropeptide expression in adult male rats. Journal of Neuroendocrinology 22:92-101.
- Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, Gorzalka BB (2009) Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology 34:2733-2745.
- Linfoot I, Gray M, Bingham B, Williamson M, Pinel JP, Viau V*. (2009) Naturally occurring variations in defensive burying behavior are associated with differences in vasopressin, oxytocin, and androgen receptors in the male rat. Progress in Neuro Psychopharmacol & Biological Psychiatry 33:1129-1140.
- Spritzer MD, Weinberg A, Viau V, Galea LAM (2009) Prior sexual experience increases hippocampal cell proliferation and decreases risk assessment behavior in response to acute predator odor stress in the male rat. Behavioural Brain Research 200: 106-112.
Further publications can be found here.
I am very grateful to several, whose guidance and training have helped me develop a unique approach to the study of stress, including Dr. Mary Dallman, Liza Soriano, Alan Chu (University of California San Francisco); Dr. Paul Sawchenko, Carlos Arias, Kris Trulock, Casey Peto (Salk Institute), Dr. Micheal Meaney, Shakti Sharma, David Aitken (Douglas Hospital Research Centre, McGill University). I am also grateful for the generous support of the Department of Anatomy and the Canadian Institutes of Health Research.
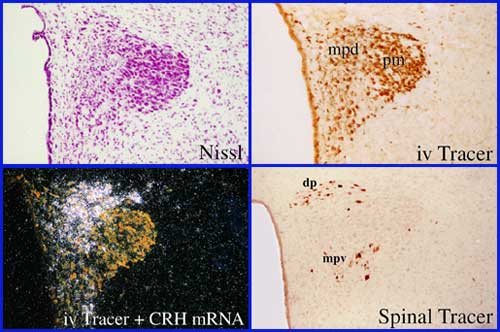
Photomicrographs illustrating some of the techniques used in my laboratory, and the connective and phenotypic features of the paraventricular nucleus of the hypothalamus (PVH), the neuroendocrine interface through which the brain regulates pituitary and adrenal output during stress. The PVH houses at least three major effector neuron classes, including magnocellular neurosecretory neurons that regulate posterior pituitary function, pre-autonomic neurons that give rise to preganglionic projections regulating both the parasympathetic and sympathetic divisions of the autonomic nervous system, and parvocellular neurosecretory neurons that express CRH among other several adrenocorticotropic (ACTH) co-secretagogues. While the PVH is arranged in a complex mosaic manner, different effector neurons can be separated based on cytoarchitectonic (Nissl), histochemical (e.g. CRH expression), and connectional (spinal- vs pituitary-projecting) criteria. Posterior Magnocellular, pm; medial parvocellular dorsal, mpd; medial parvocellular ventral, mpv; dorsal parvocellular, dp. See Swanson and Sawchenko, Ann Rev Neurosci, 1983, and Sawchenko et al., Prog Brain Res, 1996.
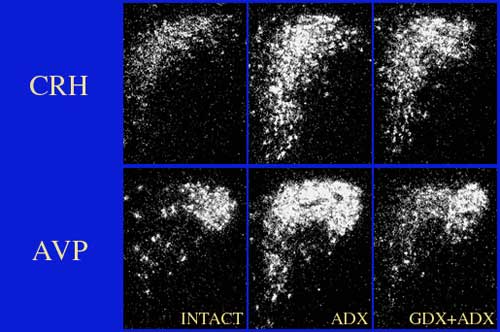
Dark-field photomicrographs of CRH and AVP mRNA expression in the PVH showing how gonadal and adrenal steroids interact on HPA function. In this case, removal of glucocorticoid negative feedback by adrenalectomy (ADX) stimulates both CRH and AVP mRNA levels within mpd neurons of the PVH. Note, however, that gonadectomy attenuates the stimulatory effects of ADX on AVP, but not CRH mRNA expression (GDX+ADX). See Viau et al., J Neuroscience, 1999.