

John Church
Professor Emeritus
Director, Graduate Program in Cell and Developmental Biology, College for Interdisciplinary StudiesBSc (Bristol)
Fellow of the Royal College of Anaesthetists (FRCA)
PhD (London)
MD (Bristol)
Email: john.church@ubc.ca
Office: 604-822-2751
Lab: 604-827-5974
Research Interests
Extra- and intracellular pH (pHo and pHi) are important physiological variables that both reflect and, in turn, influence neuronal function. Nevertheless, in comparison with other cell types, the effects of changes in pHo and pHi on neuronal activity remain relatively poorly documented. Also in contrast to non-neuronal cells, where pHi regulating mechanisms constitute a physiologically-important transmembrane signaling pathway, relatively little is known about the mechanisms that act to control pHi in mammalian central neurons. This is surprising because pHi regulating mechanisms regulate not only [H+]i and [HCO3-]i; they also affect [Na+]i, [Cl-]i and the pH in the microenvironment of cells, all of which are of critical importance to neuronal function under physiological and pathophysiological conditions. In light of this background, our research is focuses on the mechanisms that regulate neuronal pHi and their relationships to disease, in particular stroke. Recent work includes:
a) studying the effects of changes in both extra- and intracellular pH on mammalian central neuronal excitability.
b) characterizing the mechanisms responsible for the regulation of pHi in mammalian central neurons and investigating how the activities of these mechanisms can be regulated by neurotransmitters and intracellular second messenger systems.
c) investigating the effects of anoxia and ischemia on the activities of neuronal pHi regulating mechanisms, especially Na+/H+ exchangers. We are also investigating the relationships between anoxia- and ischemia-induced changes in pHi, [Na+]i and [Ca2+]i in mammalian central neurons, and assessing the contribution of alterations in the activities of pHi regulating mechanisms to these changes.
In addition, in non-neuronal cell types it is well established that Na+/H+ exchangers play a permissive role in migration and growth. We are now actively investigating whether Na+/H+ exchangers play analogous roles in neurons.
Our studies utilize electrophysiological recording techniques and imaging techniques to estimate the intracellular concentrations of Ca2+, H+ and Na+ ions, either singly or in combination, and assess the effects of pHi regulating mechanisms on cytoskeletal events at the growth cone.
- Diering G, Numata Y, Fan S, Church J, Numata M. (2013) Endosomal acidification by Na+/H+ exchanger NHE5 regulates TrkA cell-surface targeting and NGF-induced PI3K signaling. Molecular Biology of the Cell 24: 3435-3448.
- Huynh KT, Baker DW, Harris R, Church J*, Brauner CJ. (2011) Capacity for intracellular pH compensation during hypercapnia in white sturgeon primary liver cells. Journal of Comparative Physiology – B 181: 893-904.
- Huynh KT, Baker DW, Harris R, Church J*, Brauner CJ. (2011) Effect of hypercapnia on intracellular pH regulation in a rainbow trout hepatoma cell line, RTH 149. Journal of Comparative Physiology – B 181: 883-892.
- Kozoriz MG, Church J, Ozog MA, Naus CC, Krebs C. (2010) Temporary sequestration of potassium by mitochondria in astrocytes. Journal of Biological Chemistry 285: 31107-31119.
- Sin WC, Moniz DM, Ozog MA, Tyler JE, Numata M, Church J*. (2009) Regulation of early neurite morphogenesis by the Na+/H+ exchanger NHE1. Journal of Neuroscience 29: 8946-8959.
- Diering GH, Church J, Numata M. (2009) Secretory carrier membrane protein 2 (SCAMP2) regulates cell-surface targeting of brain-enriched Na+/H+ exchanger NHE5. Journal of Biological Chemistry 284: 13892-13903.
Further publications can be found here.
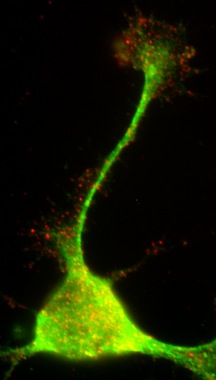
Expression of endogenous Na+/H+ exchanger isoform 1 (NHE1) in a growth cone of a mouse neocortical neuron.
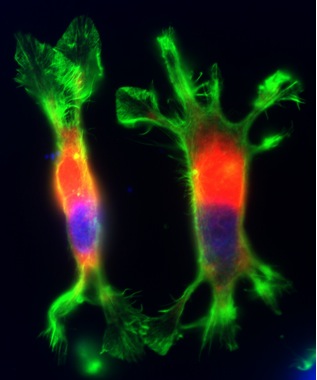
PC12 cells transiently transfected with an NHE1 mutant deficient in actin cytoskeletal anchoring.