Christopher Loewen
Professor
Cellular & Physiological Sciences
Faculty of Medicine
Other Titles
Tula Foundation Investigator (Brain Research Centre)
CIHR New Investigator
MSFHR Scholar
Member, Cell and Developmental Biology Research Group (Life Sciences Institute)
Contact
Email: christopher.loewen@ubc.ca
Office: 604-827-5961
Lab: 604-827-4160
Education
Postdoc: University College London, UK (2005)
PhD: University of British Columbia (2001)
BSc (Hon): University of British Columbia (1995)
Membrane Contact Sites and Lipid Traffic
Lipids play important roles in all cells. They differ from many other hydrophilic biological molecules in that they are hydrophobic and do not mix in the aqueous environment of cells. In this way they can function to separate the cell from the outside environment and also divide the cell into distinct compartments, called organelles. Compartmentalisation by organelles is critical for carrying out different cellular processes. For example, the integrity of mitochondria is essential to the cell to produce energy (ATP) and to regulate cell death (apoptosis).
It is also imperative that these organelles communicate with each other. Communication of the endoplasmic reticulum (ER) with other organelles, for example mitochondria, is especially important to the cell because the ER is the site of many metabolic activities including making lipids and proteins. The focus of my research is to better understand how the ER contacts, and hence communicates with other organelles through defining the molecules that mediate these contacts. Studying these membrane contact sites is important for human health and disease because their disruption can result in defective movement of lipids, cell stress and cell death. Accumulation of lipids is a factor in many diseases including atherosclerosis, Alzheimer’s disease, type 2 diabetes and motorneuron disease, but in many cases what causes this accumulation is not known. Studies in my laboratory on defining membrane contact sites and lipid traffic will help to uncover mechanisms of disease and potentially lead to novel drug targets and therapies for these disorders, thus contributing to the general health of Canadians.
Currently, my lab uses the model organism Saccharomyces cerevisiae to study this problem. We employ standard techniques in yeast genetics, biochemistry and molecular cell biology with the aim to identify the components of membrane contact sites and their roles in lipid traffic. Soon we will be incorporating sophisticated genomic and proteomic methodologies to identify new sets of genes/proteins to gain a better understanding of this process on a global scale. At present, we are investigating the role of a highly conserved protein called VAP in formation of a membrane contact site between the ER and plasma membrane. Because ER membrane contact sites are present in all eukaryotes, the genes we identify in yeast will likely be conserved in humans and will therefore be directly relevant to human cell biology. VAP has also recently been found to cause a form of motorneuron disease called Amyotrophic lateral sclerosis, and we are hopeful that our work in yeast will be relevant to this disease.
Further Reading
Levine, T. (2004) Short-range intracellular trafficking of small molecules across endoplasmic reticulum junctions. Trends Cell Biol. 14, 483-90.
A Broken Barrier Theory of ALS
• I am currently recruiting graduate students to join the lab.
Please email me with your CV attached if you are interested. •
- [1] B. P. Young, K. L. Post, J. T. Chao, F. Meili, K. Haas, and C. J. R. Loewen, “Sentinel Interaction Mapping (SIM) – A generic approach for the functional analysis of human disease gene variants using yeast.,” Dis Model Mech, p. dmm.044560, May 2020.
- [2] J. T. Chao, R. Hollman, W. M. Meyers, F. Meili, K. A. Matreyek, P. Dean, D. M. Fowler, K. Haas, C. D. Roskelley, and C. J. R. Loewen, “A premalignant cell-based model for functionalization and classification of PTEN variants.,” Cancer Res., p. canres.3278.2019, May 2020.
- [3] K. L. Post, M. Belmadani, P. Ganguly, F. Meili, R. Dingwall, T. A. McDiarmid, W. M. Meyers, C. Herrington, B. P. Young, D. B. Callaghan, S. Rogic, M. Edwards, A. Niciforovic, A. Cau, C. H. Rankin, T. P. O’Connor, S. X. Bamji, C. J. R. Loewen, D. W. Allan, P. Pavlidis, and K. Haas, “Multi-model functionalization of disease-associated PTEN missense mutations identifies multiple molecular mechanisms underlying protein dysfunction.,” Nat Comms, vol. 11, no. 1, p. 2073, Apr. 2020.
- [4] J. J. H. Shin, P. Liu, L. J. Chan, A. Ullah, J. Pan, C. H. Borchers, J. E. Burke, C. Stefan, G. J. Smits, and C. J. R. Loewen, “pH Biosensing by PI4P Regulates Cargo Sorting at the TGN.,” Dev. Cell, vol. 52, no. 4, pp. 461–476.e4, Feb. 2020.
- [5] J. J. Shin, Q. Aftab, P. Austin, J. A. McQueen, T. Poon, S. C. Li, B. P. Young, C. D. Roskelley, and C. J. R. Loewen, “Systematic identification of genes involved in metabolic acid stress resistance in yeast and their potential as cancer targets.,” Dis Model Mech, vol. 9, no. 9, pp. 1039–1049, Sep. 2016.
- [6] S. Lahiri, J. T. Chao, S. Tavassoli, A. K. O. Wong, V. Choudhary, B. P. Young, C. J. R. Loewen, and W. A. Prinz, “A conserved endoplasmic reticulum membrane protein complex (EMC) facilitates phospholipid transfer from the ER to mitochondria.,” PLoS Biol., vol. 12, no. 10, p. e1001969, Oct. 2014.
- [7] J. T. Chao, A. K. O. Wong, S. Tavassoli, B. P. Young, A. Chruscicki, N. N. Fang, L. J. Howe, T. Mayor, L. J. Foster, and C. J. R. Loewen, “Polarization of the Endoplasmic Reticulum by ER-Septin Tethering.,” Cell, vol. 158, no. 3, pp. 620–632, Jul. 2014.
- [8] A. K. Wong, J. T. Chao, and C. J. Loewen, “Barriers to uniformity within the endoplasmic reticulum.,” Curr. Opin. Cell Biol., vol. 29, pp. 31–38, Apr. 2014.
- [9] S. Tavassoli, J. T. Chao, B. P. Young, R. C. Cox, W. A. Prinz, A. I. P. M. de Kroon, and C. J. R. Loewen, “Plasma membrane–endoplasmic reticulum contact sites regulate phosphatidylcholine synthesis.,” EMBO Rep., vol. 14, no. 5, pp. 434–440, May 2013.
- [10] B. P. Young and C. J. R. Loewen, “Balony: a software package for analysis of data generated by synthetic genetic array experiments.,” BMC Bioinformatics, vol. 14, p. 354, 2013.
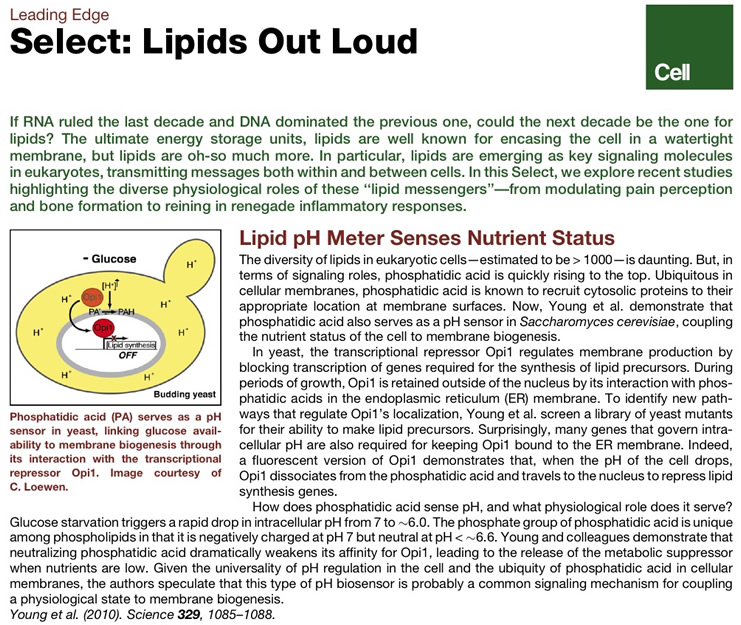
- [11] B. P. Young, J. J. H. Shin, R. Orij, J. T. Chao, S. C. Li, X. L. Guan, A. Khong, E. Jan, M. R. Wenk, W. A. Prinz, G. J. Smits, and C. J. R. Loewen, “Phosphatidic acid is a pH biosensor that links membrane biogenesis to metabolism.,” Science, vol. 329, no. 5995, pp. 1085–1088, Aug. 2010.
- [12] C. J. R. Loewen, B. P. Young, S. Tavassoli, and T. P. Levine, “Inheritance of cortical ER in yeast is required for normal septin organization.,” J. Cell Biol., vol. 179, no. 3, pp. 467–483, Nov. 2007.
Further publications can be found here.